Our Services
Dow Development Labs, LLC (DDL), a specialty contract development and manufacturing organization, provides drug product design and development services for pharmaceutical and biotech companies. We leverage our significant experience with formulation development, analytical methods, manufacturing, and clinical packaging and labeling to move topical products quickly towards regulatory approval.
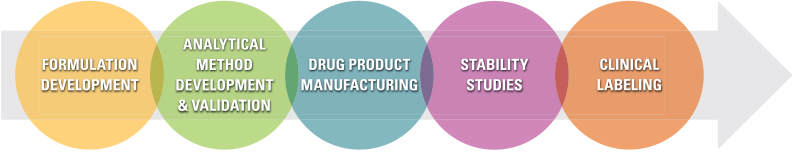
Topical Drug Product Design and Development Services