Formulation Forum
Our Blog
See Dow Development Laboratories’ FormulationForum for articles and resources on various topical drug product development topics of interest.
Determining the Minimum Concentration of Gelling Agents to Maintain Homogeneity of Suspended API in Topical Products
Using Gravitational Force to Determine the Minimum Concentration of Various Gelling Agents Needed to Maintain Homogeneity of Micronized API in Suspension Presented by Mary Magsombol-Karaan, Jonathan Behrs, Jose Viramontes, Jake Ridgway, Geneva Jump, Nkemdilim Ekomaye,...
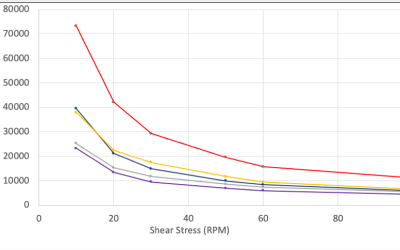
Improving the Robustness of Semi-Solid Formulations
Eliminating Operator Dependence from Gelling Agent Addition - Improving the Robustness and Processing Time of Semi-Solid Dosage Forms Without Impacting the Viscosity or Aesthetics Ruth Yu, Jason Carbol Dow Development Laboratories, LLC PURPOSE Aqueous gelling agents...
Determining the Most Suitable Gelling Agent for Topical Semi-Solid Formulations
View/Zoom in to view the PDF below PURPOSE Development of topical...
DDL AAPS 2025 Poster Presentation: Assessing the Impact of Cryo-Freezing in Different Topical Formulations
View/Zoom in to view the PDF below PURPOSE With recent pharmaceutical trends in the application of...
The Importance of Being Labeled Correctly
Among the myriad steps involved in pharmaceutical product development, clinical labeling and packaging stand out as critical components that need special attention. Clinical trials are typically the most expensive part of the already costly and years long...
The Use of Forced Degradation in Analytical Method Development
There are two times when forced degradation studies are needed when developing a stability-indicating method for the analysis of a drug product[1]. The first set of studies uses only the active pharmaceutical ingredient (API). Since the goal of the method is to...
Analytical Method Development for Semisolid Drug Products
The creation of an analytical method for the measurement of the API and degradation products in a semisolid formulation happens in parallel with the creation of the product itself. If the API is a repurposed drug substance, there is typically an existing method that...
Exploring the Use of Silicone Based Microemulsions for Formulating Aesthetically Pleasing Topical Pharmaceuticals: Enhancing Patient Compliance and Product Aesthetics
PURPOSE Formulating aesthetically pleasing topical pharmaceuticals can pose a significant challenge with low water solubility drug substances. This can lead to the avoidance of water in topical formulations in order todissolve these hard to dissolve drugs. If the...
Poster Presentation at AAPS PharmSci360
Critical Material Attributes (CMA) of Excipients in Topical Formulations: The Pitfalls of Switching Between Compendial Raw Materials Without a Full Analysis of all Critical Quality Attributes (CQA) PURPOSE With the FDA introduction of Quality by Design (QbD) many...
Controlling BCC in Non-Sterile Products – A Bacteria Receiving Increased FDA Attention
Controlling the bacteria Burkholderia Cepacia Complex (BCC) in non-sterile pharmaceutical products has received increased attention in recent years. BCC has now been classified as an “objectionable organism” by the industry and will need to be controlled...
How many pea-sized amounts are you going to use?
The FDA Inactive Ingredient Database (IID) is heavily used by formulators when developing drug products. This resource has been available since 19871 and has since informed the inactive ingredient (excipient) selections and concentrations made by formulation...
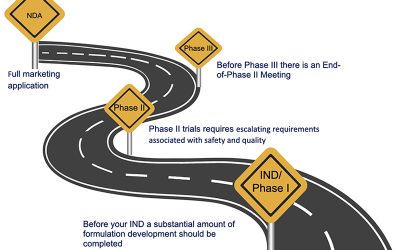
CMC Roadmap for Topical Drug Products
CMC is critical to a successful topical drug product program. Important aspects of CMC that are unique to topical products are discussed in this presentation.
Ask a Formulator: How Do You Solubilize an API That Does Not Want to Dissolve?
One of the most common issues faced during topical formulation development is difficulty in dissolving the active pharmaceutical ingredient (API) in water or other water-soluble solvent systems. When this happens, several approaches may be taken when trying to...
Scale-Up of Topical Products: Math Matters
Proper scale up of a 1 kg batch to a 1,000+ kg batch takes engineering effort to execute properly. Why is this so difficult? The short answer is because of the math. The physical forces on a semi-solid product while manufacturing a 1 kg laboratory batch vs. that of...
To Suspend or Not to Suspend
Pros and Cons of topical products containing a suspended API Prescription drug products contain an active pharmaceutical ingredient (API)1. While traditional topical drug product development focuses on dissolving the API, challenges can arise from dissolved systems....
Making pimple popping a thing of the past
Acneic skin can involve inflammation, comedones, excess sebum production and bacteria. Effective topical products for the treatment of acne must be designed with patient compliance in mind. Topical Formulations Made for Patients – Acne Products When developing topical...
Ask a Formulator: What is the Purpose and Approach to Solubility Studies?
Solubility determination of the active pharmaceutical ingredient (API) in excipients suitable for topical use is a key first step in formulation development. The objective of most topical formulations is to have dissolved API so that the API can most effectively move...
Keeping the Bugs Away
Nonsterile topical products must pass the USP/NF1 Microbial Examination of Nonsterile Products: Microbial Enumeration Tests <61> and Microbiological Examination of Nonsterile Products: Tests for Specified Microorganisms <62> and be adequately preserved. A...