PURPOSE
Formulating aesthetically pleasing topical pharmaceuticals can pose a significant challenge with low water solubility drug substances. This can lead to the avoidance of water in topical formulations in order todissolve these hard to dissolve drugs. If the formulation no longer contains water the aesthetic profile of the drug product is significantly decreased as the drug product can become heavy and greasy. A potential solution to this issue is the use of silicones as a water alternative in silicone-based emulsions. Silicones have superior scores in silkiness, freshness, and softness, while also having lower scores in irritation, greasiness, and heaviness than silicone-free emulsions. 2 However, formulating these emulsions can be problematic due to the low solubility of silicones in other solvents used to dissolve active pharmaceutical ingredients (e.g., diethylene glycol monoethyl ether and ethanol). This challenge can be overcome by employing silicone microemulsions, which are thermodynamically stable systems that typically consist of oils, aqueous components, surfactants, and co-surfactants. 3 Our study aims to explore the possibility of formulating silicone-based microemulsions for topical pharmaceuticals that are aesthetically pleasing, absent of water, and solvent containing for drug dissolution. Achieving this may enhance patient compliance and product aesthetics through the use ofhigh concentrations of silicone.
METHODS
Silicone-based microemulsions were formulated using a combination of hexamethyldisiloxane (HMDSO), diethylene glycol monoethyl ether (DGME), polysorbate 80 (PS80), propylene glycol diacetate (PGDA), and ethyl alcohol (Table 1). Through previous work it was discovered that the ratio PS80:EtOH:PGDA is critical to microemulsion success. The ratios of these ingredients were investigated with two different fixed amounts of DGME and HMDSO. The microemulsions were prepared by mixing the components in 20 mL glass vials and vigorously shaking them until a clear, homogenous solution was obtained. If a non-clear solution resulted it was determined that a microemulsion was not thermodynamically stable at those ratios.
RESULTS
There was a significant difference between the number of successful thermodynamically stable microemulsions formulated using different concentrations of HMDSO and DGME. The formation of thermodynamically stable (clear) microemulsions containing 55% w/w HMDSO and 30% w/w DGME was favored when high concentrations of co-solvent ethanol were used (Figure 1). Furthermore, the formation of thermodynamically stable microemulsions containing 25% w/w HMDSO and 60% w/w DGME was favored when high concentrations of ethanol and PGDA were used (Figure 2).
Table 1. Thermodynamically Stable and Unstable Microemulsions Formulated with Different Concentrations of HMDSO, DGME, Ethanol, Propylene Glycol Diacetate, and Polysorbate 80.
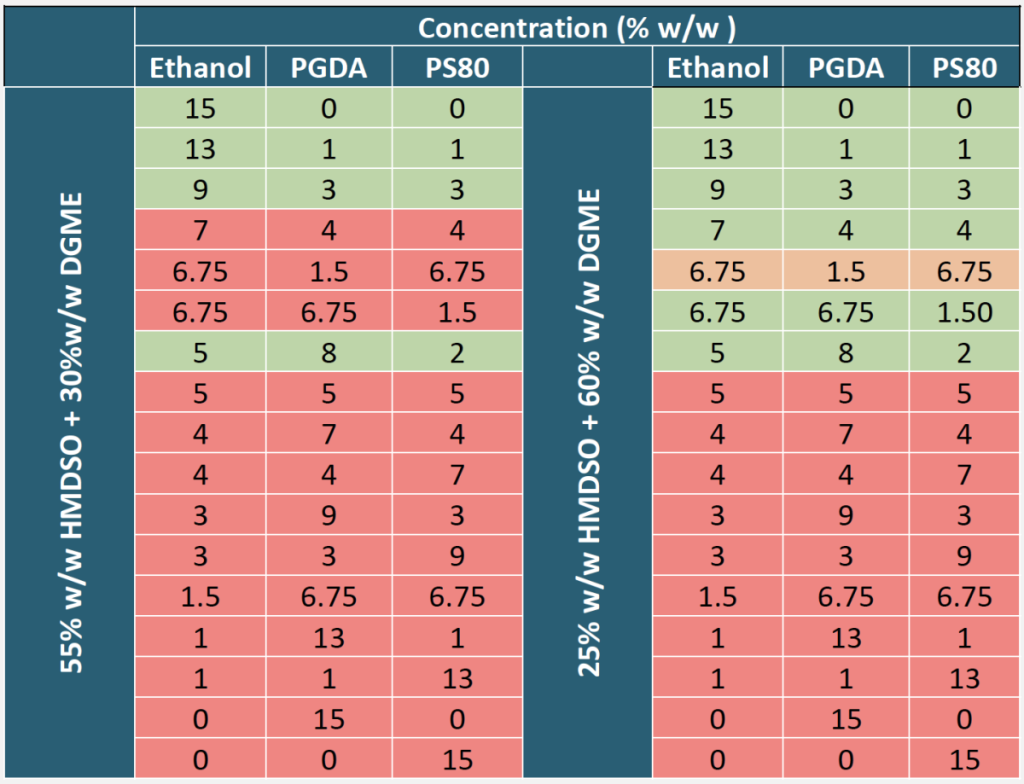
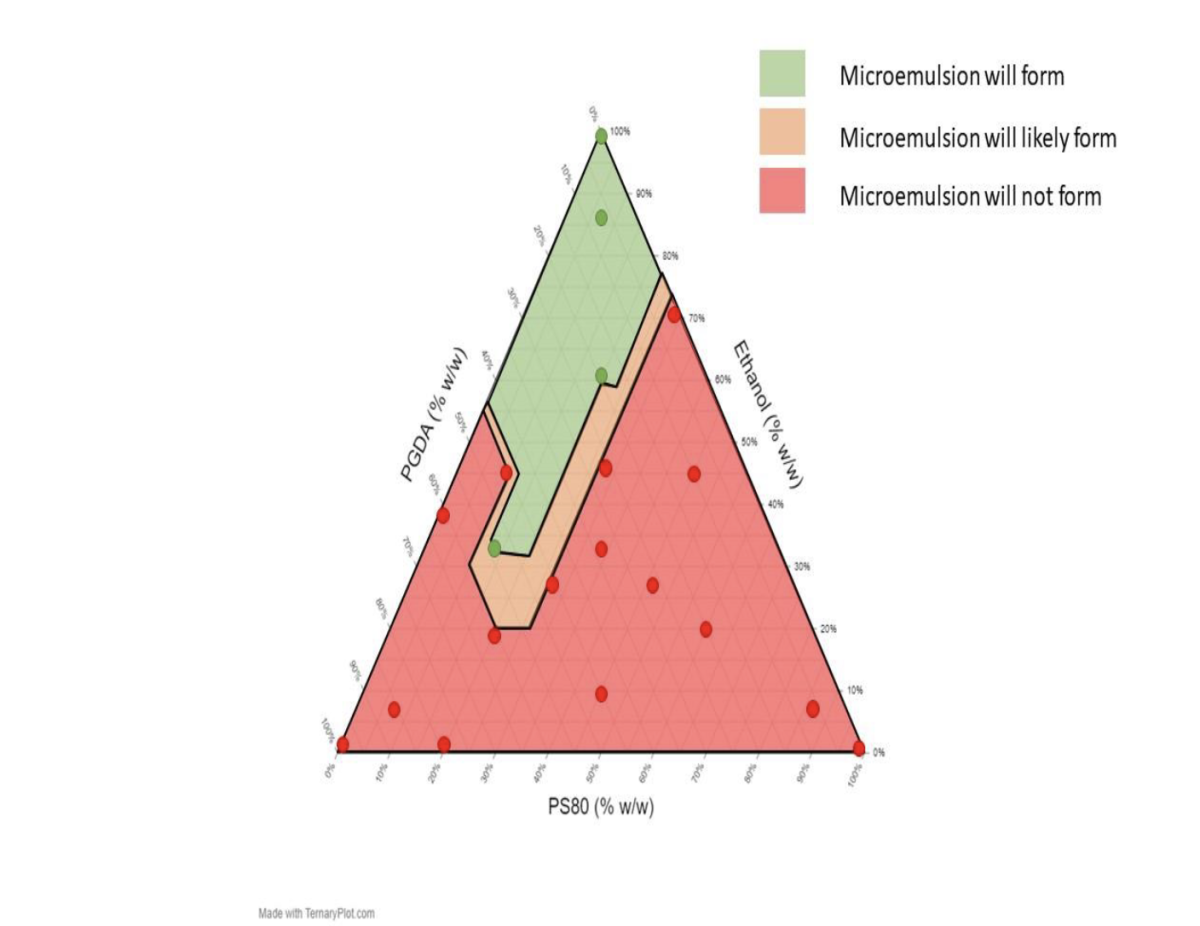
Figure 1.
Ternary Plot Showing Observed and Expected Thermodynamically Stable and Unstable Hexamethyldisiloxane Microemulsions Formulated with 55% HMDSO, 30% DGME and Various Concentrations of Ethanol, Propylene Glycol Diacetate, and Polysorbate 80 at Room Temperature.
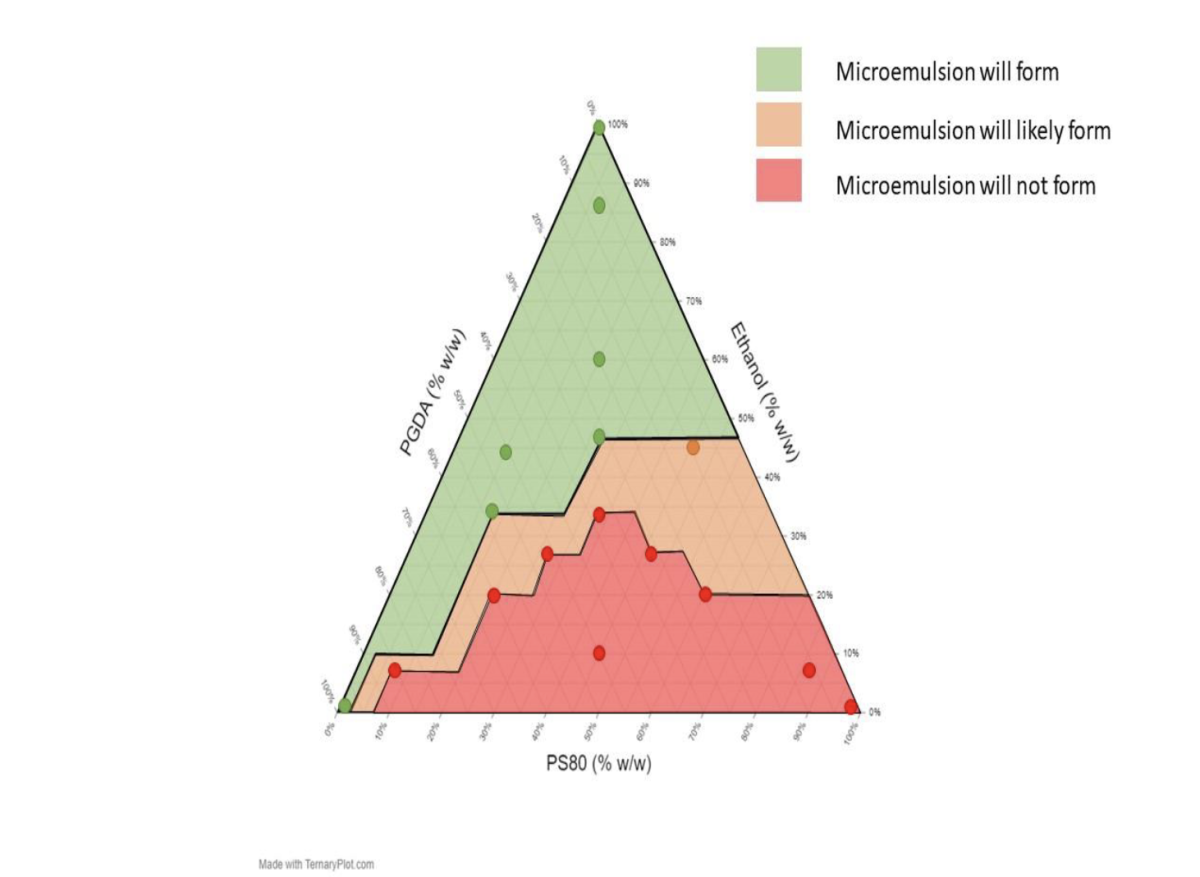
Figure 2.
Ternary Plot Showing Observed and Expected Thermodynamically Stable and Unstable Hexamethyldisiloxane Microemulsions Formulated with 25% HMDSO, 60% DGME and Various Concentrations of Ethanol, Propylene Glycol Diacetate, and Polysorbate 80 at Room Temperature.
CONCLUSIONS
Increasing Ethanol and PGDA concentrations help maintaining microemulsions stability.
- The addition of small amounts of polysorbate 80 contributes to the formulation of visually stable microemulsions with increased DGME content.
- The removal of alcohol or propylene glycol diacetate leads to decreased thermodynamic stability and phase separation, emphasizing their critical role in the microemulsion formulation.
- Overall, these results suggest that the microemulsions developed in this study may have potential applications in various fields, including drug delivery, where water as an ingredient should be avoided but a solvent such as DGME may be needed for drug substance solubility. The use of HMDSO may improve the feel of a water-free topical product while still retaining solubility of ingredients such as DGME and ethanol.
Authors: Lissette Pena Perez, Jason Carbol
REFERENCE
- SavjaniKT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:195727. doi: 10.5402/2012/195727. Epub 2012 Jul 5. PMID: 22830056; PMCID: PMC3399483
- Mancuso A, Tarsitano M, Udongo BP, Cristiano MC, Torella D, Paolino D, A comparison between silicone-free and silicone-based emulsions: Technological features and in vivo evaluation. Int J Cosmet Sci. 2022;44:514–529. https://doi.org/10.1111/ics.12800
- Suhail N, Alzahrani AK, Basha WJ, Kizilbash N, Zaidi A, Ambreen J and Khachfe HM (2021) Microemulsions: Unique Properties, Pharmacological Applications, and Targeted Drug Delivery. Front. Nanotechnol. 3:754889. doi: 10.3389/fnano.2021.754889
![M1030-02-11 Microemulsions Final Poster[82] Exploring the Use of Silicone Based Microemulsions for Formulating Aesthetically Pleasing Topical Pharmaceuticals: Enhancing Patient Compliance and Product Aesthetics](https://www.dowdevelopmentlabs.com/wp-content/uploads/2023/10/M1030-02-11-Microemulsions-Final-Poster82.png)
