Critical Material Attributes (CMA) of Excipients in Topical Formulations: The Pitfalls of Switching Between Compendial Raw Materials Without a Full Analysis of all Critical Quality Attributes (CQA)
PURPOSE
With the FDA introduction of Quality by Design (QbD) many drug product quality requirements have been placed onto developers with the expectation of better understanding their product during the development cycle. This FDA expectation was codified with the issuance of ICH Q8 (R2) in 2009. While QbD is well accepted in the industry there are still a number of potential strategies on how to de-risk a program. While de-risking systems such as critical process parameters (CPP) are well understood, and have numerous tools available such as, design of experiment (DoE), de-risking other aspects are less clear such as critical material attributes (CMA). Traditionally, CMAs are managed using specifications to ensure each raw material is meeting all the quality requirements. As a result, the use of compendial raw materials (USP/NF) with their pre-determined specifications and testing criteria, have been leaned on heavily as a means of controlling raw materials and ensuring the drug product meets its critical quality attributes (CQA). Unfortunately, over the years it has been observed that not all compendial materials have been created equal. This poster will discuss three scenarios where the reliance on compendial materials, and their associated specifications have led to products not meeting their critical quality attributes in different ways.
METHODS
As part of the topical drug product development cycle, quality by design is embraced and critical quality attributes are routinely stressed to better understand the drug product and potential points of failure. Raw materials are routinely evaluated for drug product impact. Through this evaluation, raw material issues are discovered. As a result, a library for raw materials has been generated to inventory CMA results for drug product development knowledge. The methodology was to mine this internal dataset and present the most egregious product issues resulting from changing raw material suppliers, even though the materials are meeting all specifications associated with a USP/NF monograph.
RESULTS
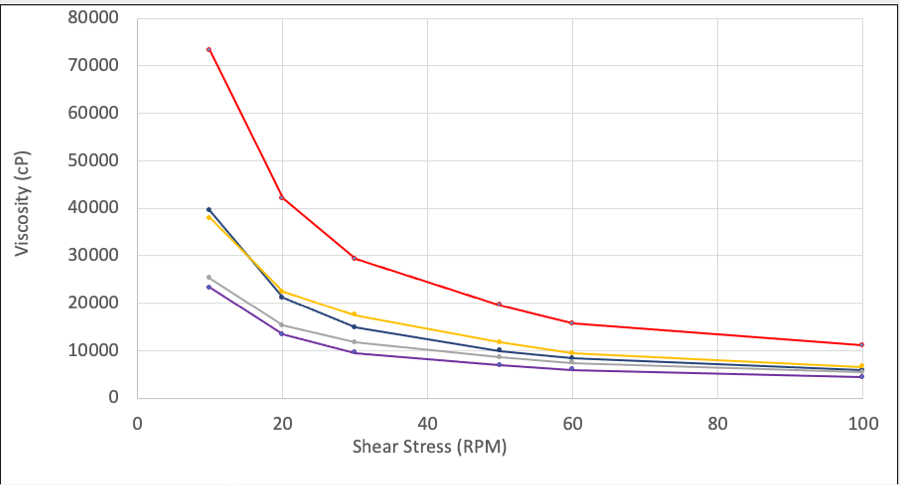
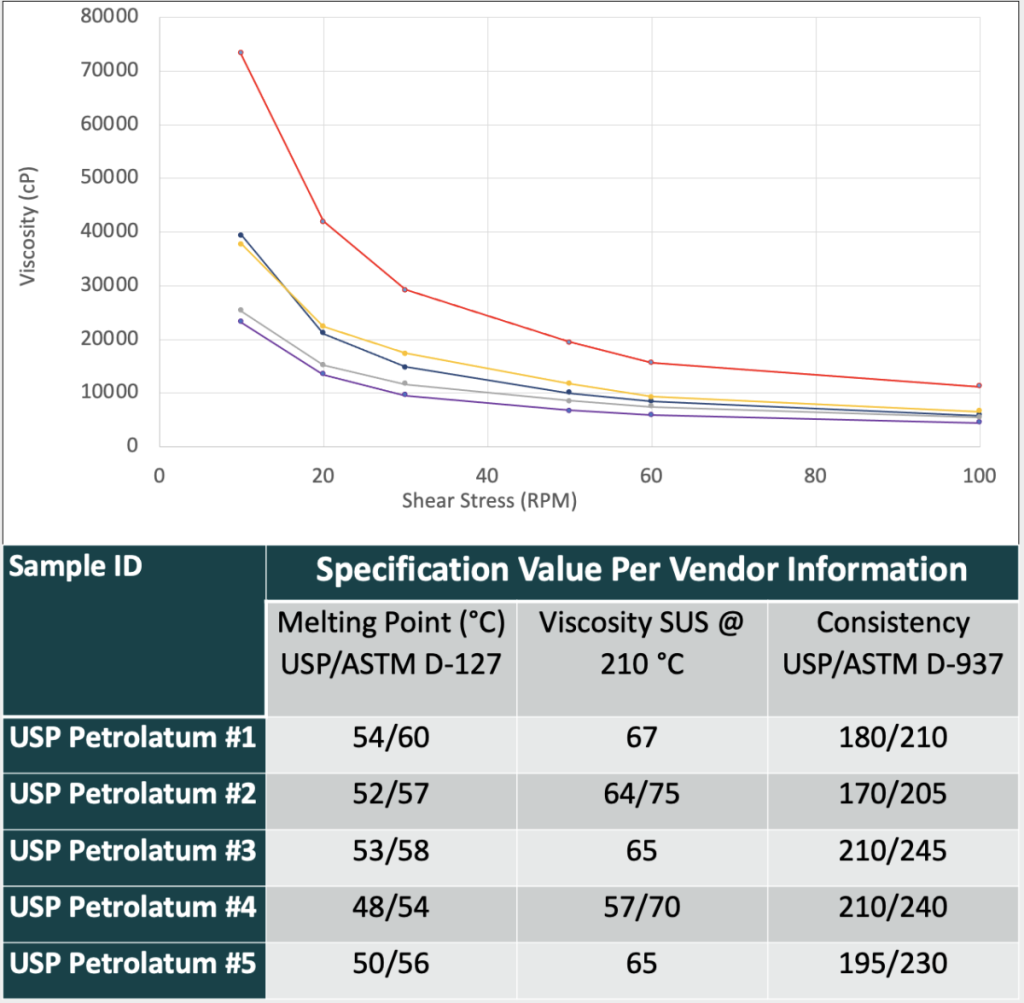
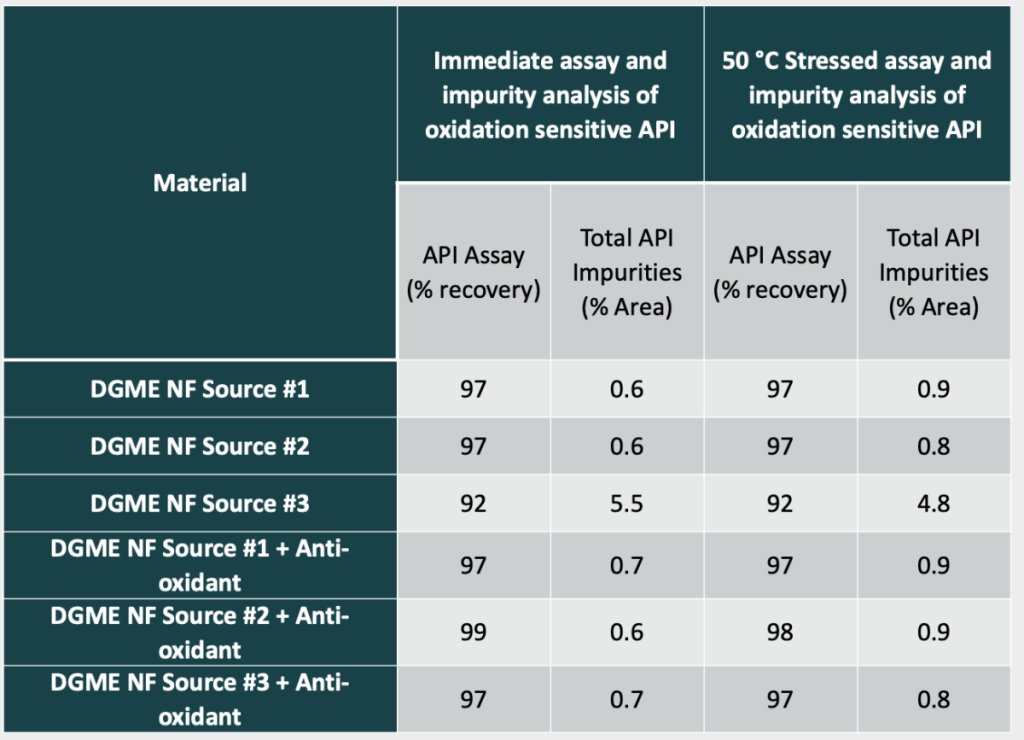
Our data-mining exercise generated three case studies. The first describes the use of USP petrolatum in topical ointments. While changing the USP petrolatum sources in topical creams does not have a significant quality impact, changing the USP petrolatum sources in a topical ointment can impact the viscosity profile of the drug product significantly. Table 1 shows how the rheological properties of the same drug product can be affected by different USP petroleum sources. A correlation between raw material specifications and resulting rheology curves were not able to predict which petrolatum sources would perform similarly. The second describes the use of diethylene glycol monoethyl ether (DGME) as a drug substance solubilizer. When screening DGME sources, it was identified that one particular DGME source contributed to more drug substance degradation than any other sources. This was a surprising result as all materials were fully compliant with the NF monograph. To further investigate this result, an anti-oxidant was included in each DGME source when solubilizing the drug substance. The inclusion of the anti-oxidant resulted in decreased degradation (Table 2). While using an anti-oxidant helped the issue, to de-risk the program this DGME supplier was avoided. The third case study involves glyceryl monostearate 40-55 (Type I). Stability issues for appearance were identified between two sources of glyceryl monostearate 40-55 NF (Type I) used. On stability, the use of one source of GMS 40-55 NF (Type I) resulted in a product that had small waxy particulates form over time while the other GMS source had no issues over the same time and longer (Table 3).
Table 3. Topical Drug Product Appearance Issues When Evaluating Glyceryl Monostearate Types.

Table 1. Resulting Thixotropic Rheology Curve of Topical Ointment Drug Product when Using Different USP Petrolatum Materials
Table 2. Resulting HPLC Assay and Impurity Values When Different Compendial (NF) Diethylene Glycol Monoethyl Ether Materials are Used
CONCLUSIONS
While much of the QbD process focuses on the drug product critical quality attributes, it is important to identify raw material critical quality attributes as well. We have shown here CQA failures of viscosity, drug degradation, and appearance in various products when raw materials are changed in each that could be considered pharmaceutically equivalent. Understanding how each raw material impacts the drug product and what effects a raw material can have on the drug product is critical to QbD principles and formulation development.
Authors: Jason Carbol
REFERENCE
[1] Lawrence X. Yu, Gregory Amidon, Mansoor A. Khan, Stephen W. Hoag, James Polli, G. K. Raju, and Janet Woodcock; Understanding Pharmaceutical Quality by Design; AAPS J. 2014 Jul; 16(4): 771–783
[2] U. S. Food and Drug Administration. Guidance for Industry: Q8 (2) Pharmaceutical Development. 2009
![Poster T1030-01-03[98] - Read-Only Poster Presentation at AAPS PharmSci360](https://www.dowdevelopmentlabs.com/wp-content/uploads/2023/10/Poster-T1030-01-0398-Read-Only-1.png)